 www.LED
www.LED
|
Please visit your German LED Growing Light Site. There you will find much more INFORMATIONS. Use the translater in 60 languages!
|
Advantages of LED growing light
The advantages of LED growing light is clearly obvious.
![]() Each home grower who illuminat his plants with the conventional sodium/HID lamps, only has to look at his electricity bill. Most of them starts here to cry, because the usual kinds of lamps use 400 to 600W and mostly they need more than one of them.
Each home grower who illuminat his plants with the conventional sodium/HID lamps, only has to look at his electricity bill. Most of them starts here to cry, because the usual kinds of lamps use 400 to 600W and mostly they need more than one of them.
![]() LEDs are producing few heat, so your water consumption will be much lower. This is also the reason, why your pumps, fans, air conditioning system ore brifly said, all your growing devices must work less.
LEDs are producing few heat, so your water consumption will be much lower. This is also the reason, why your pumps, fans, air conditioning system ore brifly said, all your growing devices must work less.
![]() As a result of the long life span of LEDs, a further saving arises. With daily use LEDs hold approx. 10 years. In the same period you would have to renew approx. 6 NDLs, to two starters and 2 condensers.... again so approx. 400,-- EUR per lamp.
As a result of the long life span of LEDs, a further saving arises. With daily use LEDs hold approx. 10 years. In the same period you would have to renew approx. 6 NDLs, to two starters and 2 condensers.... again so approx. 400,-- EUR per lamp.
![]() Larger LUX - effect. Because of the small heat LEDs can be adjusted in a short distance over the plants. Thus… small distance, results a larger density of light!
Larger LUX - effect. Because of the small heat LEDs can be adjusted in a short distance over the plants. Thus… small distance, results a larger density of light!
![]() As well some lover of the holy plant of the Hindus, the high current consumption became to fatality. The order guardians are looking for abnormal current consumer and favour the homegrowers with a visit.
As well some lover of the holy plant of the Hindus, the high current consumption became to fatality. The order guardians are looking for abnormal current consumer and favour the homegrowers with a visit.
LEDs use approx. 75% less energy than sodium lamps, with same result...
How now " less" Light can achieve the same result?
 The secret lies in photosynthesis (Greek phos - light, sýnthesis - the composition)
The secret lies in photosynthesis (Greek phos - light, sýnthesis - the composition)
or more exactly described, in the structural characteristic of the green of leaves also mentioned as chlorophyll. (Greek chloros for green and phyllon for leav)
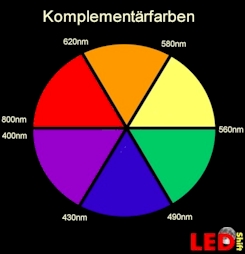
A leave appears to us GREEN because Chlorophylle can absorb low-energy, long-wave, thus red light or high-energy, short-wave, thus violet light or in addition, both colours from the white light. On the colour circle (see sketch complementary colours) green faces the colours red and violet. The chlorophyll " keeps" red and violet and we see the reflected color geen.
Why green plants mainly absorb within the red and blue range of the visible light, its easily explained:
Sunlight accommodates all colours but the blue and red part is relatively higher. According to this fact the plants adjusted themselves, in the history of the evolution, to prefer absorbing blue and red light.
 With midday sun e.g. as well when cloudy, the sunlight contains a strong blue part. With in the morning or evening sun, at shady places or e.g. also in autumn, the "Colour-mixture" of the sun looks differently. The blue part is still relatively high, however the red part already takes a considerable size.
4.5 billion years ago when our young and more and more cooling off, simmering in its own "primordial soup" but still dead planet , becomes at that time spontaneously like by accident, a breeding place of organic syntheses. And again by accident, developed from different polymers (connections from same or homogenous units) becoming by ally and stored themselves in all possible random combinations together. Some few of them developed thereby the ability to adjust and increase themselves and formed ever more complex and more stable molecule associations until the first living cell had become.
This development had taken place in such "short” time, for some a too short time interval, that many considerable scientists assume (like e.g. also Francis Crick, which became famous by the DNA Doppelhelix) that the first bacteria arrived by a spaceship or from a higher spirit at our planet.
These bacteria nourished themselves of organic molecules, which had developed from not living mechanisms. When however these nutrients went to the slope, like our fossil raw materials today, at least one group of them developed the ability to refer their food exclusively from inorganic materials, because otherwise the life would have become extinct and no humans would exist today.
That means that they began “nourished” themselves from electrons high-energy. And again, by accident, an Ur-cell included a Cyanobacteria and developed Chloroplasts - the single-cell green alga, from which probably all plants descend. Now it is perhaps more understandable, why plants adapted to use electrons by the help form the red and blue part of the sunlight.
With midday sun e.g. as well when cloudy, the sunlight contains a strong blue part. With in the morning or evening sun, at shady places or e.g. also in autumn, the "Colour-mixture" of the sun looks differently. The blue part is still relatively high, however the red part already takes a considerable size.
4.5 billion years ago when our young and more and more cooling off, simmering in its own "primordial soup" but still dead planet , becomes at that time spontaneously like by accident, a breeding place of organic syntheses. And again by accident, developed from different polymers (connections from same or homogenous units) becoming by ally and stored themselves in all possible random combinations together. Some few of them developed thereby the ability to adjust and increase themselves and formed ever more complex and more stable molecule associations until the first living cell had become.
This development had taken place in such "short” time, for some a too short time interval, that many considerable scientists assume (like e.g. also Francis Crick, which became famous by the DNA Doppelhelix) that the first bacteria arrived by a spaceship or from a higher spirit at our planet.
These bacteria nourished themselves of organic molecules, which had developed from not living mechanisms. When however these nutrients went to the slope, like our fossil raw materials today, at least one group of them developed the ability to refer their food exclusively from inorganic materials, because otherwise the life would have become extinct and no humans would exist today.
That means that they began “nourished” themselves from electrons high-energy. And again, by accident, an Ur-cell included a Cyanobacteria and developed Chloroplasts - the single-cell green alga, from which probably all plants descend. Now it is perhaps more understandable, why plants adapted to use electrons by the help form the red and blue part of the sunlight.
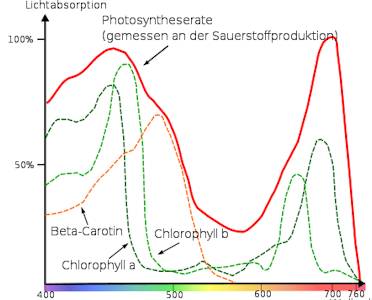 Angel man tested the ability of the chlorophyll, within the different ranges of the light spectrum, to see where oxygen is produced of light energy. In this experiment he was sending a beam of light through a prism to separate its spectrum. This he projected it on a thin alga thread, on which it had laid oxygen loving bacteria.
Angel man tested the ability of the chlorophyll, within the different ranges of the light spectrum, to see where oxygen is produced of light energy. In this experiment he was sending a beam of light through a prism to separate its spectrum. This he projected it on a thin alga thread, on which it had laid oxygen loving bacteria.
The result shows it clearly that the optimal range for photosynthesis is with long-wave red light, as well as in the short-wave blue light. Within the range of green and yellow the photosynthesis rate drops to nearly 0%.
The grasp for the sunlight:

The chlorophyll molecule is a green plant dye (pigment molecule) that serves the plants as a kind “antenna", with which they can take up the energy of the sun, in order to link carbon dioxide and water to carbohydrates. This colouring material consists of two components: The blue- green chlorophyll A and the yellow- green chlorophyll b. Chlorophyll A (molecule of the reaction center) is responsible for the energy conversion and chlorophyll b however, has gotten by mother nature purely the challenge to collect the light. Chlorophyll A is with 410 to 430nm and around 662nm most actively and chlorophyll b “rebuild" with 453nm and 642nm the most photons.
Which light brings the plant now more... RED or BLUE?
The redox potential of the chlorophyll (measure for the ability of a material to deliver electrons):
Many will assume here that high-energy blue light puts reliably more energy to the plants at the disposal than the low-energy red light. However, this acceptance is wrong. More can be sometimes also too much. This applies also with the photosynthesis. One can introduce oneself in such a way:
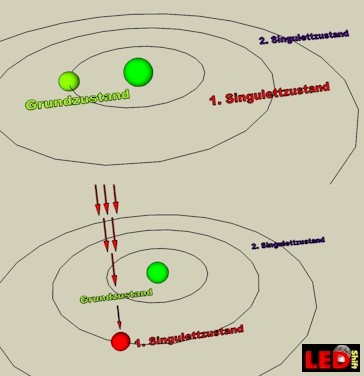 In our sketch we show an atom with his nucleus and a circling electron (in reality chlorophyll possesses a pair of electrons). If no light meets chlorophyll the electron, it is in the GROUND STATE, thus on the lowest energy level.
In our sketch we show an atom with his nucleus and a circling electron (in reality chlorophyll possesses a pair of electrons). If no light meets chlorophyll the electron, it is in the GROUND STATE, thus on the lowest energy level.
If now a RED light particle meets with 660nm (photon) the electron of a pigment molecule, the contained energy is absorbed and it begins to vibrate so strongly that it jumps on the 1.Singulett-state (S1). It is as it were “brake out”. In this condition it is higher-energy than in the ground state and can be easily delivered and go on his journey by the electron flight conveyor.
For this it would be still important to stress that only in this S1-state (high redox potential) the electrons can be easily delivered.
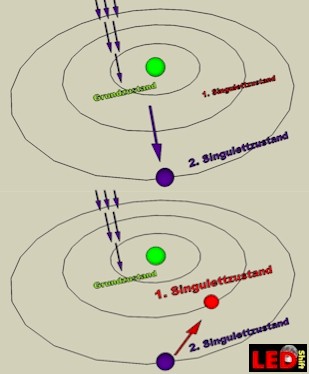 With BLUE light the case looks completely differently. The electron gets so much energy of the high-energy blue photon with 430nm that it gets catapulted up to the “S2-Orbit". In this singulett-state it is for photosynthesis not directly usable. Only, it is owed to the fact, that this condition last only 10 minus 12 seconds, that the electron drops back to the S1 state and give off energy in the form of e.g. heat or light. Only then the electron is ready for the electron flight conveyor .
With BLUE light the case looks completely differently. The electron gets so much energy of the high-energy blue photon with 430nm that it gets catapulted up to the “S2-Orbit". In this singulett-state it is for photosynthesis not directly usable. Only, it is owed to the fact, that this condition last only 10 minus 12 seconds, that the electron drops back to the S1 state and give off energy in the form of e.g. heat or light. Only then the electron is ready for the electron flight conveyor .
Now it is clearly evidently why the absorption of blue light brings the plants no direct advantage. But if the naturally red light is missing the plants also can use it.
RED ore BLUE Light?:
Plants need both colours. Only both wavelengths provide a good root formation and a healthy spreading and length growth. The high-energy blue light is for young plants thus in the growth phase very important and red is essential for the flowering phase. The best mixing proportion depends of the kind of the plant. Parsley e.g. loves a high blue part (approx. 90%). The holy plant of the Hindus however, loves one red-blue portion of approx. 5:1 and many garden plants feels with a relationship of 8:1 well. That blue portion can vary depending upon plant and growth requirements between 1% and 20%. The best is, one makes a few experiments with coloured LEDs and different homogenous seedling . The individual LEDs are cheap and it makes a lot of fun to observe the positive reactions of its plants. More information to this topic ' s, further down on this side.






 The sun side of the earth becomes in one second 1,7 x 10 high 14 KW/H!!! sun power. 30% of the solar radiation gets fortunately at present time still reflected. If however in the future the polar caps (white reflects very well) become smaller, this value will drastically sink. 47% are absorbed by the atmosphere (e.g. ozone), as well of the water and land masses and is used for the heating up of earth, water and air. The remaining 23% are used for weather, thus for the movement of air (e.g. trade winds) and body of water (e.g. gulf stream) and for the evaporation of water. If someone here perhaps would ask: How can the temperature of the earth remain always so beautifully constant on the basis such energy quantities? ; it is said that the surplus energy is released fortunately still in the form of radiant heat into space. If mankind however blows further so much carbon dioxide into air, then this radiant heat more and more gets reflected by the terrestrial atmosphere and a threatening heating will come up (greenhouse effect). |
It indicates, how much solar power on 1m ² per second vertical radiates. It amounts to 1.400Watt/m ². Only 46% of this energy are used for the light within the visible range. But this value is not very relevant for us, because it refers to a place above the terrestrial atmosphere. Here with us on the earth's surface it depending upon degree of latitude, to altitude, weather and air purity approx. 400 to 800W/m² inclusive UV and radiant heat.
Here we know seen why LED growth lamps so energy-saving and efficient. Into this optimal approx. 800W radiation by m² covers also all other wavelengths, which the plant cannot evaluate. If one e.g. illuminates its flowers only with green light, it is, with the “eye” of a plant total DARKNESS!!! For us however, the color green is, the brightest color.
 Perhaps another example helps of a better understanding:
Perhaps another example helps of a better understanding:Imagine a table fully covered with 400 delicious sausage. There is salami, ham, different spread and so on... In order to arrange the whole table more delicious, we also used two salads for decoration.
How now a cow would see this table? Exactly, mmm... 2 salads!
We assume now that each kind of sausage and the lonely working salads, represent a wavelength. (The salads would be the wavelengths 430 and 660). How now a plant would see this table. Exactly, mmm... 430 and 660...! :o).
Thus it behaves e.g. also with the sodium-vapor lamps. A sodium-vapor lamp, one says, is good for the plant breeding, since she produces tremendously MUCH light. The stupid is that the plant of only can use approx. 10-20% of the spectrum, the rest is only wasted energy in the form of heat and other wavelengths.
Who e.g. illuminates its plants with a Halogenspot with 30.000 lm, and believes, now my favourites must feel comfortable and grow, with so much light like mad!, may be pleased only about a high electricity bill, but the plants brings this light nearly nothing. Halogen emitters nearly no light which is usable for the photosynthesis of a plant. Here you can see nicely how much energy is wasted. Green and yellow is for the plant "COMPLETELY DARKNESS" |
The summer sunlight, outside at noon brings approx. 100,000 lux. In the shade only approx. 10,000 lux. Behind the window is it only 3,000 to 2,500 lux and 2 meters away of the window only 1/4 of the original light intensity is available. In the middle of the room the values usually is around 300 lux. In addition, it constitutes a large difference, which " position" (N, S, O, W) the windows has, whether curtains are used and which colour the walls are painted. Dark walls reflect nearly no light. Generally one can say that plants with a texture on the leaf need more light then plants without texture. Plants you don’t placed by the window needs additional light.
 If you use only red light you cause an excessive length growth at the expense of the form growth of the plant (Photomorphogenese). One speaks here of a etiolation of the plant. Large sprout are formed, which cannot often carry their own weight. Sometimes this effect is desired.
If you use only red light you cause an excessive length growth at the expense of the form growth of the plant (Photomorphogenese). One speaks here of a etiolation of the plant. Large sprout are formed, which cannot often carry their own weight. Sometimes this effect is desired.
Red light affects also the bloom and the seed production of the plant. With high red part during the dark phase (night rest), the bloom phase can be e.g. extended, which usually is unwanted. The proportionately of red and dark red light is responsible when the bloom of the plant begins. E.g., if a plant should form seeds, it should be illuminated with only little red light, because most seeds develop later to male plants. If the plant is placed by the window, you can use only red light because the blue part at this place is sufficient. The Photorezeptor of plants for red light is called Phytochrom.
 If you illuminates only with blue light it leads to compression and formation of thicker leafs. With some plants it can come also to axillary bud. In addition, these axillary bud can be desired. Therefore plants, which were raised with high blue part, mostly has a compact stature with strong sprout. The more blue lights it is present, the further opens also the gap openings of the plant whereby the metabolism is accelerated. The Photorezeptor for blue light is called Cryptochrom.
If you illuminates only with blue light it leads to compression and formation of thicker leafs. With some plants it can come also to axillary bud. In addition, these axillary bud can be desired. Therefore plants, which were raised with high blue part, mostly has a compact stature with strong sprout. The more blue lights it is present, the further opens also the gap openings of the plant whereby the metabolism is accelerated. The Photorezeptor for blue light is called Cryptochrom.
Humans & Plant: What plants need is also healthy for humans.
This chapter does not have much to do with plants, however we find it fits here exactly here. Scientists found out that the human skin positively reacts exactly to these two wavelength. 107 patients with easier to moderately acne vulgaris were submitted to a test with LEDs. They got radiated 15 minutes daily with portable LED lights. The patients did not know with which light it were illuminated. After 12 weeks of the active treatment with the blue and red light, 76% of the photo therapy group was ameliorated with acne inflammations. One found out that blue light (415nm) initiate the skin to produce peroxide which kills the acne bacteria. However red light (633nm) stimulates the defense protection against the skin cells and leads to the fact that the skin regenerates better, lets acne wounds heal faster and the skin visibly recovers. Thus who lets itself often illuminate with its plants together, soon gets a skin like a Babypopo…: o) more under... British Journal of Dermatologgy
Who particularly loves its plants, should switch on a warm white light with a high addition of infrared portion, so approx. 1-2 hours before the turning off. This " Afterburn" Effect is for a long time well-known, because the better plant growth or yield is clearly proven with these Metode. Why the plants react in such a way is unknown, because for the Photosyntese this infrared light is nearly useless. One assumes that the plant gets a control signal: "the sun goes down soon!”
Plants need as we humans a recovery in darkness. The metabolism with plants works cyclically, were certain materials from the leafs are getting removed and/or others are brought. If you use LEDs this night rest is not so necessary, because the plant in not so stressed as if you use a light with all wavelengths. But however it’s the night rest is recommended. (e.g. Afterburn effect). 3 to 6 hours is here sufficient depending upon plant type and growth phase.
Also the Co2 content of air and the temperature can accelerate or slow down the photosynthesis. The optimal temperature lies with approx. 25-33°C and the Co2 content with approx. 0,1 Volums%. Greenhouses increase their yield e.g. by CO2-gassing. Who often speaks with its plants, in short distance, supplies her additional with Co2. : -)
We were often asked if UV light is good for the plants? For this the following is to be mentioned.
There are three kindes of UV light:
UV-C (100 - 280nm), UV-B (280 - 315nm) and UV-A (315 - 400)
![]() UV-C light becomes fortunately absorbed in the atmosphere. Artificially produced it is used for the sterilization. This “light” is useless for the Photosynthesis.
UV-C light becomes fortunately absorbed in the atmosphere. Artificially produced it is used for the sterilization. This “light” is useless for the Photosynthesis.
![]() UV-B light is biologically effective and damages the DNA of the plants. Thus again uselessly, at least for most plants. Scientists produced recently found out that if one illuminates a salad with small quantities of UV-B, it in the outside layers of the cells polyphenole connections (Antioxidantien). These help to block the UV-B radiation, which would damage the plant. Some these connections are red and belong to the same group which for the color of the berries and the apple skin are responsible. Food at Antioxidantien is rich has for humans differently advantages and to cause e.g. an improvement of the brain function up to a slowing down of aging.
UV-B light is biologically effective and damages the DNA of the plants. Thus again uselessly, at least for most plants. Scientists produced recently found out that if one illuminates a salad with small quantities of UV-B, it in the outside layers of the cells polyphenole connections (Antioxidantien). These help to block the UV-B radiation, which would damage the plant. Some these connections are red and belong to the same group which for the color of the berries and the apple skin are responsible. Food at Antioxidantien is rich has for humans differently advantages and to cause e.g. an improvement of the brain function up to a slowing down of aging.
![]() UV-A light is harmless. Clorophyll A however begins to “fell comfortable” itself only off approx. 400nm, and this is violet light.
UV-A light is harmless. Clorophyll A however begins to “fell comfortable” itself only off approx. 400nm, and this is violet light.
In our left linkbar you find more infos about this topics
Most manufacturers indicate the light-current (lumen, lm). Lm is the entire light achievement of a shining object, independent of its radiation angle ore wavelength. Like we described above, lumen is an USELESS unit for growth lamps.
Luminous intensity = Candel (cd):
Here the data in luminous intensity (CD),would be already more meaningful because also the reflected beam angle is here considered.
If one e.g. uses a 400W sodium-vapor lamp without reflector, which boasts e.g. with 50.000 Lummen, come with the calculation " hot" 3,900 CD out.
A 20W!!! Ledspot with 25° reflected beam angle (LED Spot/25° 20W) radiates with 6.700 CD!!! That means 20 times les energy with nearly double luminous intensity!
But information’s about the wavelength the luminous intensity (CD) gives no statement.
1cd=12,566lm and cd = lm:sr. Here we see that cd is calculated from lm and if lm is useless cd is it as well.
Lux, Lux indicates the density of light on a surface (Lux=lm/m ²). One could mean that these numbers sound already better, because as reference value mostly the midday sun with 100.000 lux or the cloudy skies with 10.000 lux is presented. As in the example cow-plant described, the sunlight contains all wavelengths and most are for the plant useless. Thus lux are also not a good reference value. Lux gets calculated from lm... that means useless as well!
But how we can compare now growing lamps?
A good starting-point would be the light spectrum:
Wavelength in Nanometer (nm)
Chlorophyll A is most active with 410 to 430nm and around 662nm. " The light collecting pigmente" Chlorophyll b collects with 453nm and 642nm the most photons. If you knows the spectrographic analysis of your growth lamp you can at least say if she is working in the optimal range. But how much energy your lamp puts into the different wavelength, it is not shown.
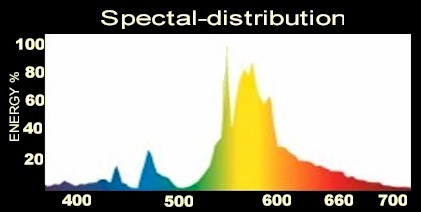
 Spectral energy distribution in %
Spectral energy distribution in %
These data would be the clearest and simplest after our judgement. In one view you can see how much energy your lamp puts into the respective wavelength. In the sketch the energy distribution of a metal-vapor lamp is shown. Nearly the entire energy is wasted in yellow and green. Unfortunately these data are rather rarely available.
Color temperature K
The color temperature is only with white light a meaningful indication not for growing lights. Some will object here: why… my metal-vapor lamp does radiate with white light? This is correctly, but here an example. Your growing light possesses a color temperature, we says of 5.800K. In our table under color temperature, the midday sun is e.g. indicated with 5.800K. This is nothing else, what color a black body, with the temperature of 5.800°K would have. Red e.g. has approx. 2500K and blue has approx. 10.000K.
Mol, Einstein and the Avogadro constant:
One Einstein is on mol and is also the numerical value of the Avogadro constant in mol-1.
Mol is a quantity measure. One mol (mol) of a substance with the relative molecular mass M contains exactly M gram of this substance.
1 mol of a substance contains always 6 X of 10 23 molecules. One calls this number also Avogadro´ constant.
PAR or PhAR und microEinstein (µE):
The Photosynthetically Active radiation is the range in the spectrum of the solar radiation, which can be used by photo-synthetically active organisms.
The unit PAR indicates the millionsten part of Einstein by m ² and second. 1 microEinstein (µE) are 6 x 10 to the 17 photons by m ² and second.
However, we determined that the term PAR refers to all photo-synthesizing organisms and again covers the entire spectrum (like e.g. green light), which within the plant communities (inclusive photosyntetisied bacteria such as Cyanobakterien - blue algae, which process also green light) are usable. Also again, not the optimally unit.
PUR:
The photo-synthetically usable radiation (Photosynthetically Useable radiation) or also called PURE. This unit expresses the connection between the potentially photo-synthetically usable radiation (PAR) and the individual radiation use for photosynthesis. A pur- sensor trims the incident radiation in the kind, as it corresponds to the middle spectral sensitivity of the photosynthesis of the Green plants. The pur radiation is indicated in energy current density (W/m ²). It has the appearance that PURE represents the best unit for growth lamps. But this unit is not so easy to understand and all physicists we asked about this topic, not really could help us.
So we tried by ourselves to trace this PUR/W/m ².
Chlorophyll molecule absorbs approx. 45 photons by second. That means the photosynthesis rate can not increased by irradiation of higher light. If the light saturation is reached one could switch still dozens of growth lamps to it, the result, up to a high electricity bill, remained the same.
The energy content of a light quantum lets itself calculate from the Planck formula. I.e. the energy of a light quantum is a function of the frequency or wavelength of the light:
Energy of a photon = hv = h x (c/wavelength)
h is the famous Planck constant (6.624 x 10 to the minus 27Erg x second) and C the speed of light approx. (3 x of 10 to the 10 centimetre per Secunde).
For RED light with a wavelength of e.g. 700nm the energy 6.77 x 10 amounts to the minus 20 calories per quantum or 41 kilocalories per Einstein (1 Einstein is 6.02 x 10 to the 23 quanta). If every light quantum moved one electron thru the photo system we need 164 kilocalories. That would be approx. 190W/m ², if we do not have calculated wrong... :o)
This number relies however also approx. on the solar constant. If 1400W/m ² energy only contains 15% of red (622-770nm) light it would be around 210W/m².
Who would like to start now some trials with his plants by him self, or needs individual LEDs for seedlings or additional side lighting, here some favorable proposals:


Tis site youalso find in our LEDshift NEWS
| AGB Ledshift | LED Function | LED Producer | LED Distributor | LED Events | LED Company Entry | LEDS | Sitemap | Contact | |
|
|
| © Markus Kottas, Heldenberg Ltd.2018 |






























